Abstract
Introduction: Methotrexate is an antifolate that has been an essential part of regimens for acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL), when it is used in high-dose (HD, ≥ 1 g/m2) to overcome the blood-brain barrier. Nephrotoxicity is the major toxicity of HDMTX, inducing critical organ damage and death if not early detected and treated. Therefore, these regimens are usually administered in the hospital, with intravenous (IV) hydration, drug level, urine pH monitoring, and MTX-guided leucovorin rescue. Although some factors for drug-induced acute kidney injury (AKI) have been pointed and recommendations are published, the reality is that in resource-poor settings, HDMTX is usually administered in an outpatient clinic and without drug monitoring. The exact incidence of AKI after MTX in these conditions was not reported in adults yet. In this study, we intend to summarize our toxicity data after outpatient administration of HDMTX without drug monitoring. We aim to find risk factors and describe their outcomes.
Methods This is a retrospective nested case-control study conducted at an academic medical center in Sao Paulo, Brazil. Patients from 16 years old and above with ALL and NHL who received at least one outpatient infusion of HDMTX without drug level monitoring between Jan/2010 and Jun/2020 were included. Most patients with ALL received a 6-hour infusion, while those with NHL received a 2-hour infusion in a day clinic, along with intravenous hydration, urine alkalinization, and leucovorin rescue. Patients were followed outpatient by a multidisciplinary team. Cases were those patients who developed AKI by KDIGO definition or with delayed MTX clearance (serum MTX level > 0.1 mM after 72h from drug infusion), when clinically suspected. A nested cohort of controls was composed of patients who did not develop the complication, sampling from a larger cohort of patients who received HDMTX. Cases and controls were randomly matched 1:2 for the employed protocol only. Hematologic and liver toxicity were graduated according to CTCAE v5.0. Conditional logistic regression was used to find baseline risk factors.
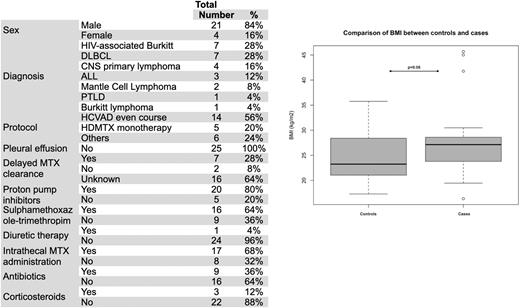
Results A total of 404 patients received at least one HDMTX infusion, with 102 not fulfilling the inclusion criteria. Among 302 patients included, 840 infusions were performed (median 2 infusions/patient, range 1-10). Most patients had a diffuse large B-cell lymphoma (DLBCL) (37%), Burkitt lymphoma (BL) (19.5%), or ALL (18%) diagnosis, followed by other NHL subtypes (25.5%). Most subjects received HDMTX within a course of Hyper-CVAD (HCVAD) or monotherapy (61%). Hospitalization occurred in 8.6% of infusions during the MTX nadir, with 4.6% needing intensive care, being febrile neutropenia the most common complication (63%). Twenty-five patients presented AKI after HDMTX administration, corresponding to 3% (95% CI 2-4.4) of MTX infusions and 8.3% (95% CI 5.5-12.1) of patients. Characteristics of cases are summarized in Table 1. Univariate analysis for finding pre-treatment factors related to AKI after HDMTX were age>44 y (OR 3.2 [95% CI 1 - 9.7], p=0.045), body surface≥1.76 m2 (OR 3.4 [95% CI 1.2-9.9], p=0.039), BMI≥23.8 kg/m2 (OR 3.5 [95% CI 1.2-9.9], p=0.02), baseline creatinine clearance (OR 0.96 [95% CI 0.93-0.99], p=0.012), thrombocytopenia (<150x109/L) (OR 4.7 [95% CI 1.7-13.1], p=0.002). Multivariate analysis for adjusting such factors found that only BMI is independently related to AKI after HDMTX in our setting (OR 3.8 [95% CI 1.2-11.8], see figure 1). Among those patients with AKI, 9/25 had their MTX serum level measured at the AKI, with delayed MTX clearance in 7/9. HIV-associated BL was more frequent in cases (18 vs. 6.8%, p=0.03). Concerning remaining toxicities, grade 3 or more thrombocytopenia, neutropenia, and hyperbilirubinemia were found in 36%, 64%, and 36%, respectively. 22/25 needed hospitalization, with infection diagnosed in 23/25 e need for dialysis in 5/25 cases. Death after AKI occurred in 56% (14/25).
Conclusions Our data showed a similar rate of AKI after HDMTX to that reported in the literature, even without drug monitoring. However, patients who developed AKI in our cohort fare worse than expected, with more hospitalizations and death. Higher BMI was associated with MTX-induced AKI in our cohort, suggesting a differential drug clearance and the need for specific guidelines for obese patients.
Disclosures
Pereira:Janssen: Research Funding; Zodiac: Honoraria; Astrazeneca: Honoraria, Research Funding; Bayer: Research Funding; Celltrion: Research Funding; Sandoz: Honoraria, Research Funding; Eusa: Honoraria; Takeda: Honoraria; Libbs Farmacêutica: Honoraria, Research Funding; Roche: Research Funding. Rocha:Amgen: Speakers Bureau; Pfizer: Speakers Bureau; Takeda: Speakers Bureau; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal